Research
We strive for a detailed understanding of the interactions between living cells and nanostructures with respect to cell behavior, cell physiology and cell mechanics.
This knowledge will allow us to develop novel nanoscale devices with applications in biology and medicine, such as:
- the development of nanowire devices that can interact with cells with minimal cell perturbation (e.g. for injection, nanobiopsy, mechanosensing)
- the development of nanowire tools which can control the cell behavior (e.g. with respect to differentiation, migration, proliferation, stimulation….)
We are working the following projects:
1) Nanowires for neural implant design
We have shown that, in vitro, nanowires promote neurite outgrowth and prevent glial cell growth, which makes them promising nanomaterials for designing neural interfaces that support neurons steadily over time while limiting the formation of a glial scar.
In 2007, we pionneered the culture of neurons on nanowire arrays, see Hällström et al. 2007.
In 2013, we showed as a proof of principle, that nanowires could be used for acute recordings of single units in the rat brain, see Suyatin et al. 2013.
We are now working on understanding the interactions between nanowires and neurons/glial cells, see for instance Piret et al. 2013, Piret et al. 2015
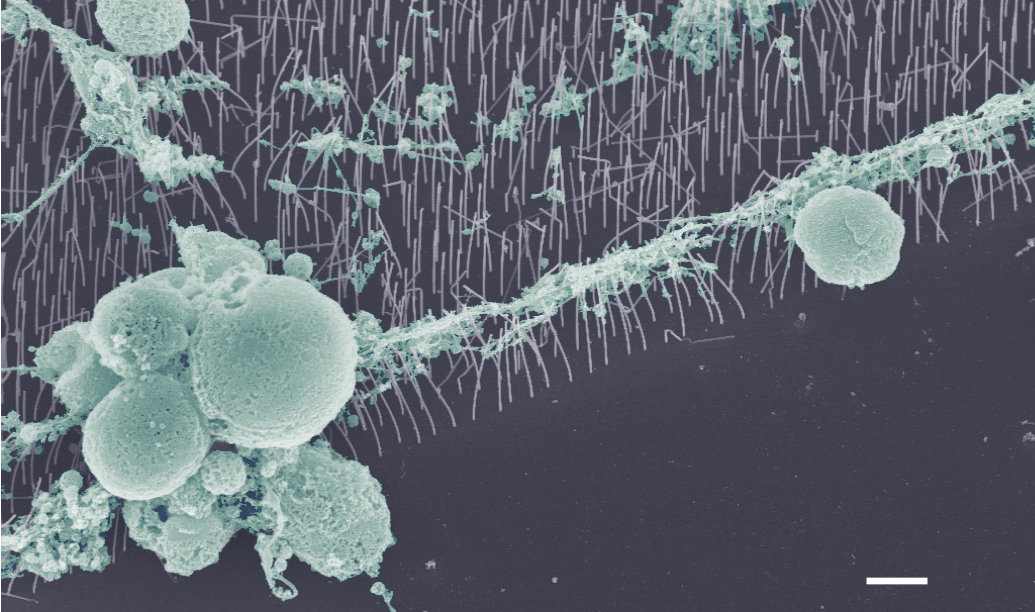
SEM image of retinal cells cultured on vertical nanowire arrays
Collaboration: Maria-Thereza Perez, Division of Ophthalmology, Lund University
Past collaboration: Jens Schouenborg, Neuronano Research Center, Lund University
2) Nanowires for cellular sensing
We measure cellular traction forces by culturing cells on top of an array of vertical nanowires and measuring the nanowire deflection induced by the cells. By tuning the dimensions of the nanowires, our method can measure forces ranging from tens of picoNewtons to tens of nanoNewtons.
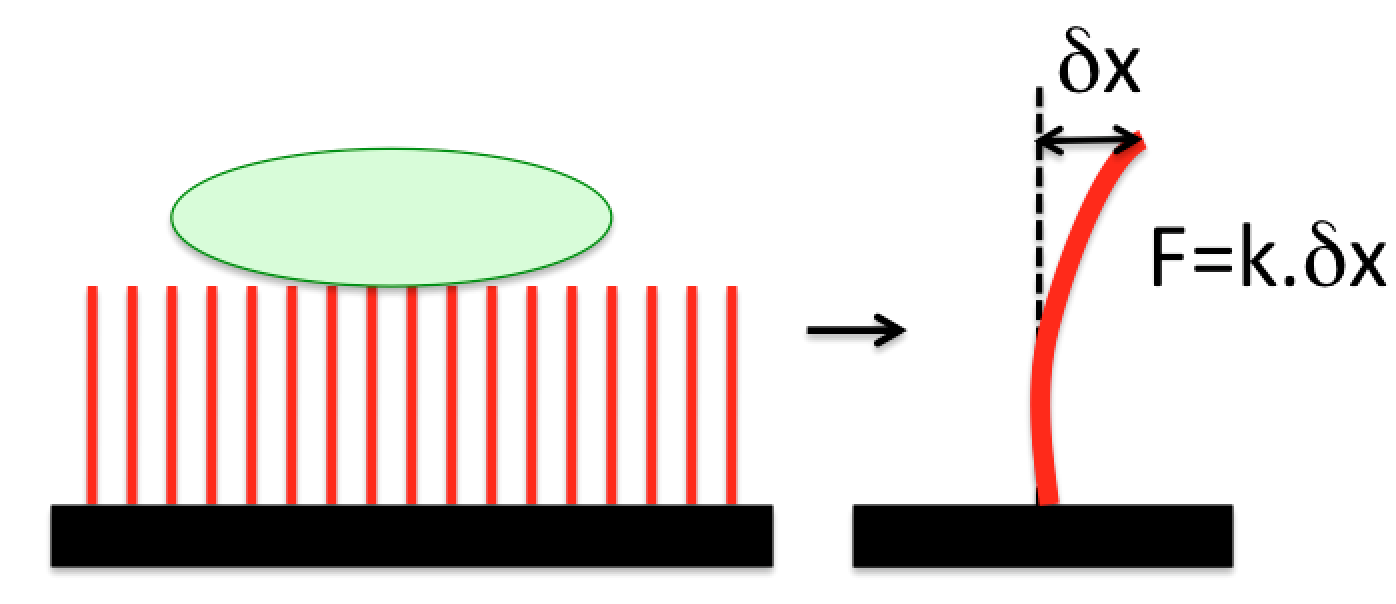
Schematics of the traction force measurement method
See, for instance Hällström et al. 2010.
Collaborations: Kalle Åström, Centre for Mathematical Sciences, Lund University; Dan Hessman, Division of Solid State Physics, Lund University; Stina Oredsson, Department of Biology, Lund University
3) Nanosafety
We take advantage of the great control we can achieve over nanowire geometrical and optical properties to investigate the effects of nanowires on organisms.
Thanks to the great control over nanowire dimensions, we have investigated the effects of the sole nanowire diameter in Daphnia magna, a model system for ecotoxicity studies. We have found that 40 nm diameter nanowires translocate through the Daphnia intestinal epithelium, in contrast to 80 nm diameter nanowires, which stay in the gut, see Mattsson, Adolfsson et al. 2016.
To facilitate our nanosafety studies, we have developed nanowires with inherently photoluminescent GaInP segments for direct nanowire identification in biological samples, see Adolfsson, Persson et al. 2013. These barcode nanowires are compatible with super resolution microscopy imaging, which will be used to obtain detailed information on the interactions between nanowires and biological tissue, see Oracz, et al. 2017.
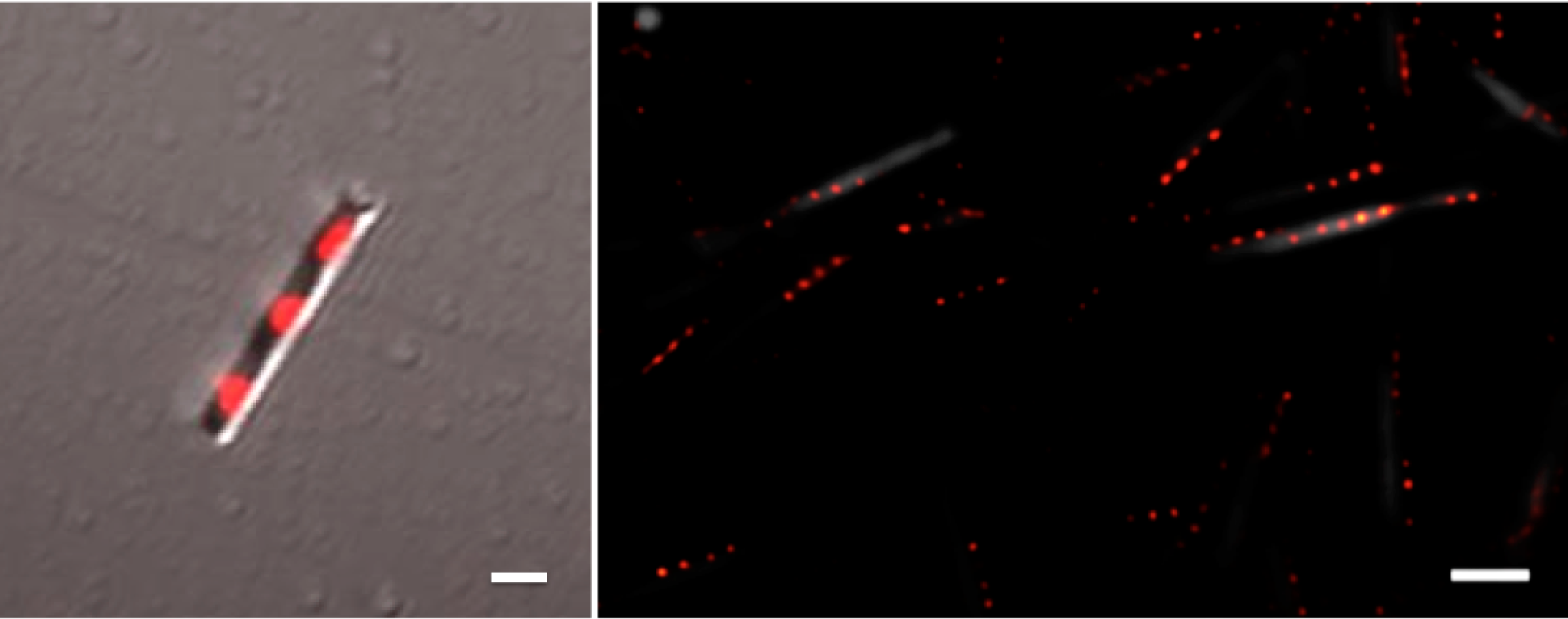
Left: Confocal fluorescence microscopy image of one GaP-GaInP nanowire (gray: scattered light, red: GaInP luminescence). Right: Ground state depletion microscopy image of GaP-GaInP nanowires with 4 GaInP segments. Scale bars 1 µm
Collaborations: Udo Häcker, Department of Experimental Medical Science, Lund University; Magnus Borgström, Division of Solid State Physics, Lund University; Tommy Cedervall, Department of Biochemistry and Structural Biology, Lund University; Stefan Hell, Max Planck Institute for Biophysical Chemistry, Göttingen.
4) Investigation of supported lipid bilayers on nanowire arrays
Membrane curvature affects protein and lipid distribution and plays an important role in cell function. To investigate this, we use model membranes on nanowire arrays, which impose a controlled membrane curvature on the nanoscale. The bilayer properties are assessed using fluorescence microscopy and FRAP (see Dabkowska et al. 2014 and Dabkowska et al. 2015).
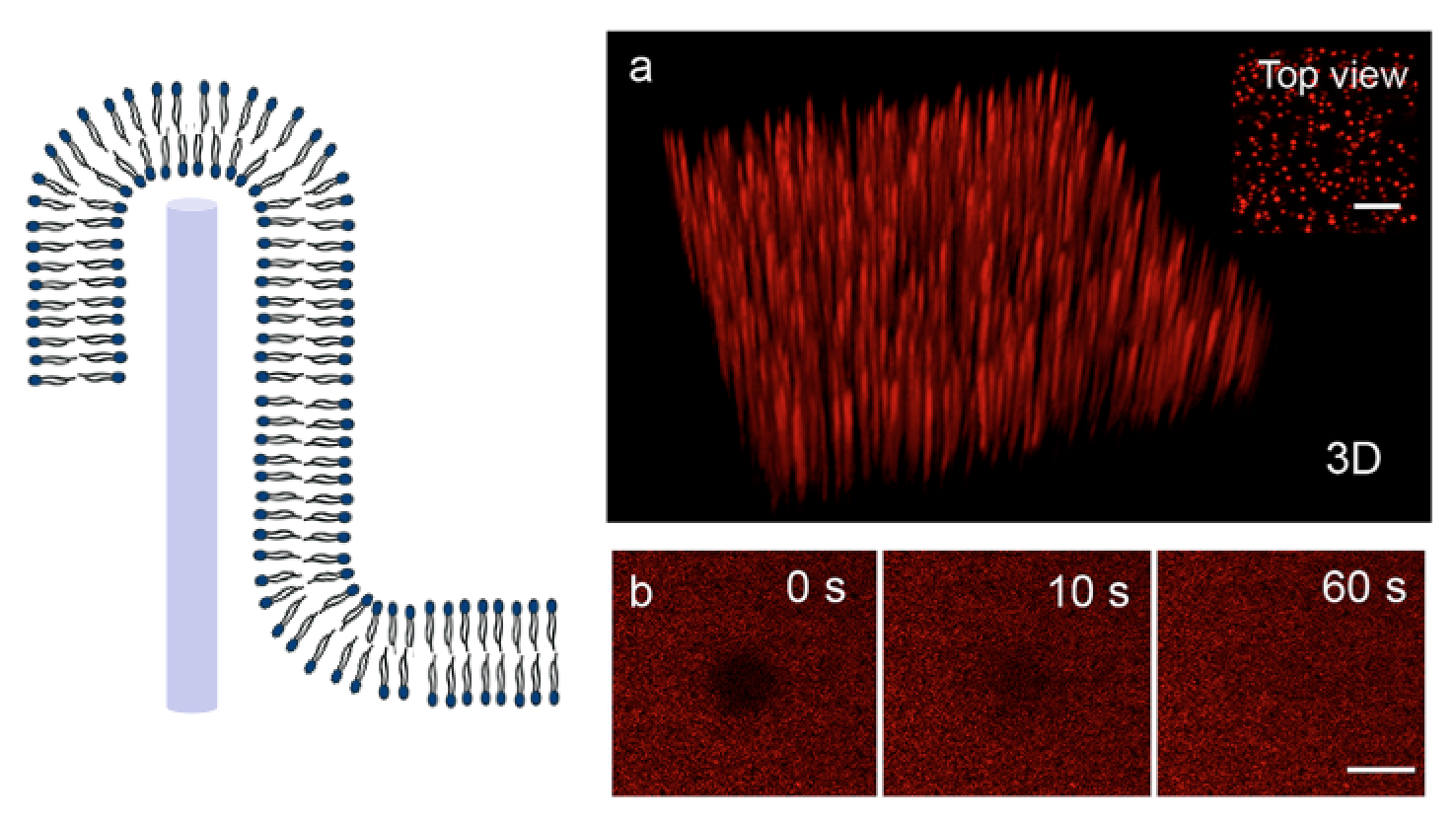
Supported lipid bilauer formation on vertical nanowire arrays
Collaborations: Heiner Linke, Division of Solid State Physics, Lund University; Tommy Nylander, Division of Physical Chemistry, Lund University.
5) Steering cell behavior using nanowires
By tuning the geometry of vertical nanowire arrays, we can affect cell proliferation, motility and morphology.
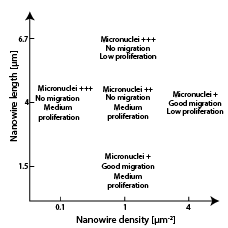
See for instance, Persson et al. 2013 and Persson et al. 2015.
Collaboration: Stina Oredsson, Department of Biology, Lund University
Funding